When a coating is applied to a surface, it typically undergoes two phases: drying and curing. Drying is the evaporation of solvents, water, or other ‘carriers’ used to make it easier to apply the coating. Curing is the actual cross-linking and hardening of the coating, usually through new chemical bonds forming between molecules. Drying and curing are two different processes, which is why they are discussed separately in the Drying, Curing, Circulation and Ventilation guide. There are many different coating systems (e.g.: epoxies, high solids alkyds, latex, varnishes, lacquers, drying oils, powder coatings, etc.) all with slightly different mechanisms of drying and curing. This guide will primarily focus on the oxidative (air drying) process. For a little more technical information, a good place to start is the ‘Drying Oil’ Wikipedia page.
What happens when a coating or paint is applied?
Most paints and coatings are comprised of two main ingredient segments: solids/resins and solvent/carrier. The diagram below, in general, shows what happens when a coating is applied. There are three steps in the process.
Step 1: Application and Evaporation
The first step is the application of the coating to the wood or other surface. Overall, the liquid on the surface is very much the same as it is in the can with small portions of the resin separated by the solvent. When exposed to the environment and spread in a thin layer, the solvent begins to evaporate and the chains get closer together, but still remain individual chains.
Step 2: Drying
Once the solvent has evaporated, the surface is considered DRY. In this second step, all the molecule chains are tightly bunched together, but still not chemically bound to one another. At this point, the coating will feel dry (usually) and usually takes only a few hours. Theoretically, more solvent could be added at this point to redissolve all the chains back into the liquid (not in actuality; however, as some curing has begun). All the twisted molecule chains are entangled and wrapped up around one another, thereby behaving like a solid coating; however, the coating has not fully cured. In fact, some coatings, like shellac, stop curing at this step. The molecule chains are all tangled up and act like a solid coating, but can be easily redissolved by reintroducing alcohol as a solvent.
Step 3: Curing
The third and most important step is the actual curing of the product. Because all the molecule chains are stuck so close together, the individual chains begin to cross-link with one another and form new, long molecules. This curing mechanism will vary depending on the specific chemistry that is used, but the result is the same. The coating becomes a single, VERY large molecule that is hard and cannot be redissolved in the same solvent it was originally delivered. The curing process will start to take place as soon as the proper conditions are met, but can take many days or weeks to reach completion.
Auto-Oxidation
All Waterlox products, including PURE Tung Oil, cure through a mechanism called auto-oxidation. In this mechanism, oxygen molecules from the air help to initiate the chemical reactions that allow the oil and resin molecules to cross-link. Initially, it is very easy for oxygen to find unreacted resin molecules, so the initial cure is quick, especially at the surface level. As the un-cured chains are cross-linked and consumed, it is harder for an oxygen molecule to find its way deeper into the film, therefore full cure can be many days or weeks.
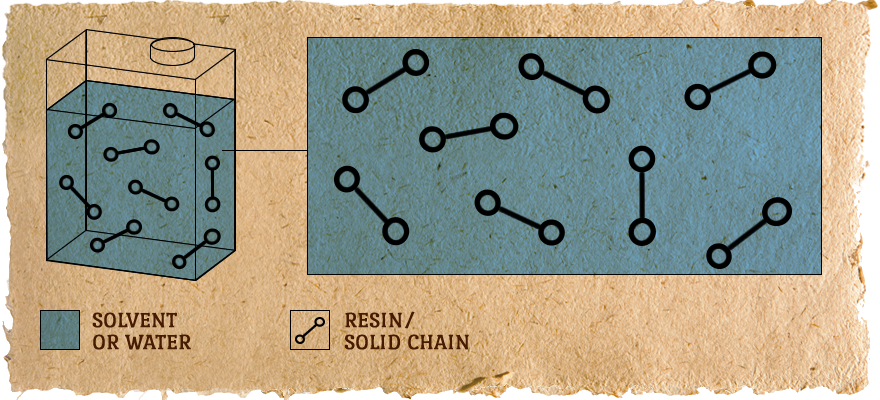
In the Can:
Resin/solids are short chains dissolved in solvent or water.
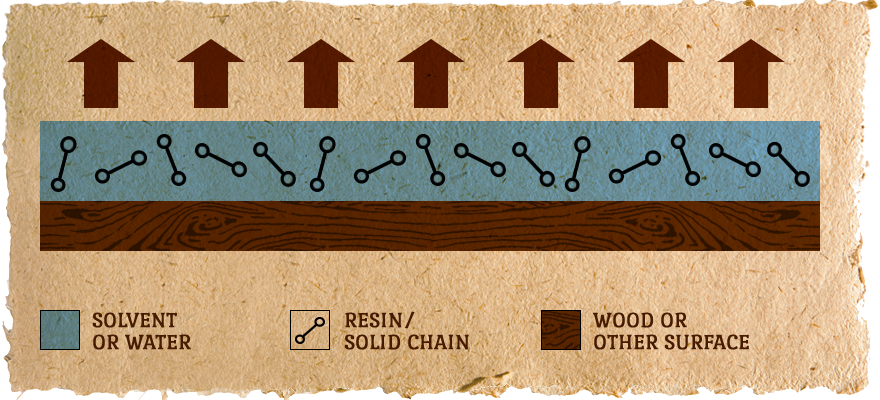
Coating is Applied to the Surface:
DRYING occurs as the solvent/water evaporates.
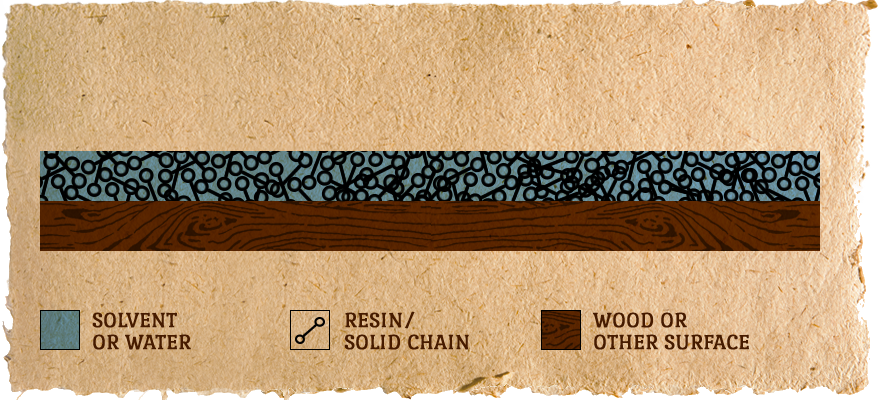
Surface is DRY:
Almost all of the solvent is gone and the resin/solids are forced very close together, but they are still separate molecules. Usually a few hours.
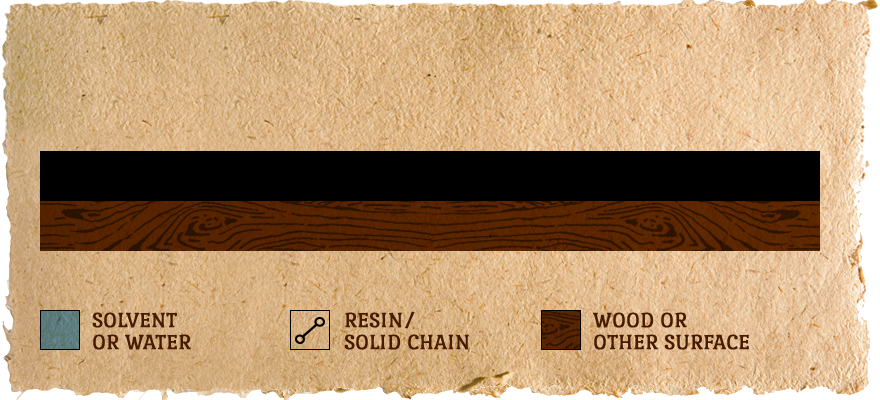
Surface is CURED:
Resin molecules are forced together and form actual chemical bonds between them to form one very large molecule. Usually a few days.
Curing of Waterlox Products
Waterlox products all cure through auto-oxidation. Good air circulation is critical to help the oxygen molecules find their way to any uncured molecules. Below is a little more information on each product line and how they cure.
ORIGINAL, H2OLOX®, MARINE, and URETHANE
These products are very conventional coatings in that they are lower in solids (<45%) and they behave very similar to the diagram above. Each product line uses metallic driers to help pull oxygen into the coating to speed up the curing process. As the solvent evaporates, oxygen molecules are pulled in and free radicals are created. These free radicals find uncured double bonds and form cross-links between polymer chains and the free radical is consumed and the oxygen is released back out of the coating. Once cured, these will form a hard, transparent surface.
TRUETONE® and UTOS
These products are very high in solids (>80%), so there is much less solvent to evaporate and the wet film will not shrink much. These chains are already really close together, but they are much smaller segments. This makes them easier to apply, but require more cross-links to be formed after application. Applying them with a buffer or by rubbing them into the surface makes a much thinner layer, so it is easier for the oxygen to reach all the molecules. The thin layers also eliminate frosting or wrinkling of the coating. If a thick layer is applied, the coatings will cure into a thick, frosted and softer layer.
PURE Tung Oil
PURE Tung Oil is 100% solids and is usually considered to be the best ‘naturally occurring’ drying oil. Tung oil is called a drying oil because it will actually cure when exposed to oxygen. This process can take weeks, but if left out on a surface exposed to air, it will eventually form a soft, frosty film. Cutting the PURE Tung Oil in solvent or applying very thin coats will yield good clear layers, but as with the UTOS and TRUETONE®, it will not form a transparent, hard film. By modifying the tung oil molecule, Waterlox is able to enhance these natural curing properties to create almost all of our product lines for superior protection.






